560
Views & Citations10
Likes & Shares
Results: Results showed a moderately positive correlation between an ASCVD risk score and Fibro scan E results, with the findings being statistically significant. However, there was no significant difference in A.S.C.V.D. Risk score in both fatty and non-fatty liver groups as well as steatosis and non-steatosis groups.
Conclusion: Identifying NAFLD in type II DM patients may facilitate better cardiovascular disease risk estimation with practical management implications. Moreover, identifying people with NAFLD would highlight a subgroup of diabetic patients who may benefit from more intensive preventative treatment and cardio-protective measures to decrease their risk of future CVD.
Keywords: Non-alcohol fatty liver disease, ASCVD Risk Score, Fibro scan, Type II DM, NAFLD fibrosis score
Approximately 60% of T2DM patients have NAFLD [11,12]. Furthermore, patients with T2DM and NAFLD have a higher risk of cardiovascular disease than patients with T2DM alone, suggesting a potential synergistic increase of cardiovascular disease risk in patients affected by both conditions [13,14]. Recent data indicate that the presence of NAFLD in T2DM is strongly linked to increased CVD risk independently of the metabolic syndrome. However, this hypothesis needs verification through more extensive studies. When assessing disease severity and risk of progression to cirrhosis, it is helpful to divide NAFLD into NAFL and NASH via their histologic differences. In NASH, hepatic inflammation is present in contrast to NAFL, which involves only steatosis [15]. A NAFLD Activity Score (NAS) assigns numerical values to different histologic measures of steatosis, inflammation, cell injury, and fibrosis in one approach used to define the extent of the disease's severity. The cumulative score classifies patients as having NAFL, borderline NASH, or fully developed NASH. Commonalities in the pathophysiologic changes between patients with NAFLD and T2DM cause an increase in cardiovascular risk. These changes can include proatherogenic lipid alteration, elevated thrombotic factors, insulin resistance, low-grade inflammation, and changes to the microbiome. With the framework above, this study has examined the CVD risk in NAFLD patients with T2DM.
MATERIALS AND METHODS
The Department of Medicine at G.S.V.M Medical College tested cardiovascular atherosclerotic disease in NAFLD patients with T2DM from December 2019 to October 2021 using an observational cross-sectional study. Subjects included fifty-five T2DM patients screened as prospects for the study, including patients receiving treatment inside the outpatient department (OPD), an affiliated diabetes clinic, and affected individuals admitted to a hospital. All participants signed informed consent forms, and the G.S.V.M. Medical College Ethics Committee approved the study protocol.
Patient Selection
The study group consisted of patients above the age of 18 years old with T2DM. There were numerous factors we set as exclusionary criteria. These included all any cause of chronic liver disease, including viral infections (i.e., positivity for Hepatitis B/C) other than NAFLD, any history of hepatotoxic drug use (i.e., anti-tubercular treatment), and patients who were classified as significant alcohol consumers based on the recommendation guidelines set forth by the American Association for the Study of Liver Disease (A.A.S.L.D.). In patients without liver disease, the A.A.S.L.D. Recommends that women consume no more than one standard drink per day and that men consume no more than two standard drinks per day, with a standard drink containing 14 grams (g) of ethanol (EtOH) [16]. Moreover, any patients with congestive heart failure (CHF) were also excluded. All patients underwent detailed questioning about their past medical history and social history, as well as a full clinical physical examination. The findings were logged in a specially prepared proforma. All the patients underwent extensive lab testing using both blood and urine analyses. Tests conducted and values obtained included an electrocardiogram (E.C.G.), hemoglobin (Hb), total lung capacity (T.L.C.), diffusion lung capacity (DLC), and erythrocyte sedimentation rate (E.S.R.), urine microscopy, HbA1c, random blood glucose, alanine aminotransferase/aspartate aminotransferase (SGPT/SGOT) ratio, serum bilirubin (total and differential), kidney function test, total serum protein, albumin, globulin, ultrasonography (U.S.G.) of the whole abdomen, and transient elastography.
Transient Elastography Fibro scan
The severity of NAFLD in patients was assessed using U.S.G. abdomen for confirming fatty liver, and transient elastography controlled attenuation parameter (C.A.P.) value for hepatic steatosis grading and E (kPa) value for liver fibrosis categorization as shown in Tables 1 & 2. In addition, N.F.S. was also calculated using age, blood sugar, B.M.I., platelet count, albumin, and AST/ALT ratio for correlation. Diagnostic criteria for T2DM assessment included an HbA1c level >6.5 and two-hour plasma glucose >200mg/dl after 75gm glucose load. Cardiovascular risk was assessed using 10-year A.S.C.V.D., calculated using free online software M.D. Calc.
Data Analysis
Statistical analysis was accomplished using Statistical Package for Social Survey version 28 (S.P.S.S. Inc., Chicago, IL). The data obtained were analyzed using the chi-square test, Kruskal Wallis test, and Fisher exact test. A p-value
RESULTS
Demographic Characteristics
The mean age of all patients was 55.24±10.09 years (Range 35-78 years). The maximum number of patients were from the 51-60 age group (32.7%, n=18), and the minimum number of patients were from the 71-80 age group (3.6%, n= 2). Majority of patients were female (52.7%, n= 29).
Physical Examination Characteristics
The mean height (cm), weight (kg), waist-to-hip ratio (WHR) and BMI (kg/m²) of patients were 157.60±8.28, 61.78±12.12, 1.00±0.10 and 24.69±4.21 respectively. The distribution of BMI of patients were as follows: 3 (5.5%) of the patients had BMI of 2, 12 (21.8%) had BMI of 18.5-22.9 kg/m2, 14 (25.5%) had BMI of 23.0-24.9 kg/m2, 20 (36.4%) had BMI of 25.0-29.9 kg/m2, 4 (7.3%) had BMI of 30.0-34.9 kg/m2 and 2 (3.6%) had BMI of 35.0-39.9 kg/m2.
The mean systolic blood pressure (S.B.P.) and diastolic blood pressure (DBP) were 132.76±16.44 mmHg and 76.18±11.63 mmHg.
Glucose and HbA1c levels
The mean postprandial blood sugar was 229.25±71.69 mg/dL, the mean fasting blood glucose was 147.15±40.15 mg/dL, and the mean HbA1c was 9.05±2.81%.
A.S.C.V.D. risk score & Assessments
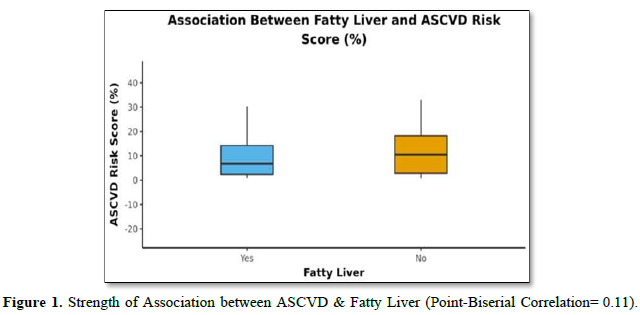
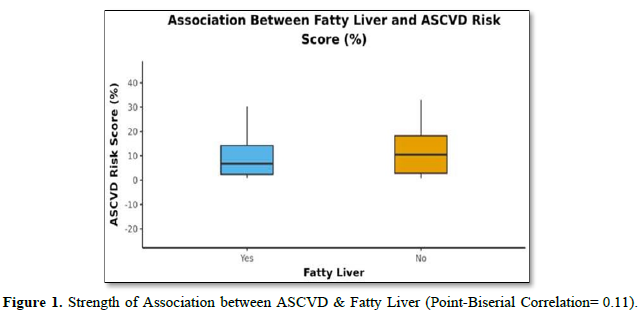
The mean A.S.C.V.D. Risk score was 11.16±11.58. In males, the mean was 14.11±13.54, and in females, it was 8.52±8.93. Forty-two (76.4%) of the patients had fatty liver, and thirteen (23.6%) had no fatty liver, as shown in (Table 3). The mean A.S.C.V.D. risk score in the fatty liver group was 10.43±11.25, and the mean A.S.C.V.D. risk score in patients without fatty liver was 13.53±12.78. There was no statistically significant difference between the groups regarding A.S.C.V.D. risk scores (W = 234.000, p = 0.446) or association (Point-Biserial Correlation of 0.11), as shown in Figure 1.
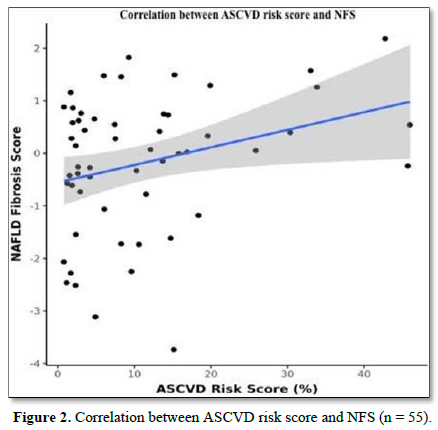
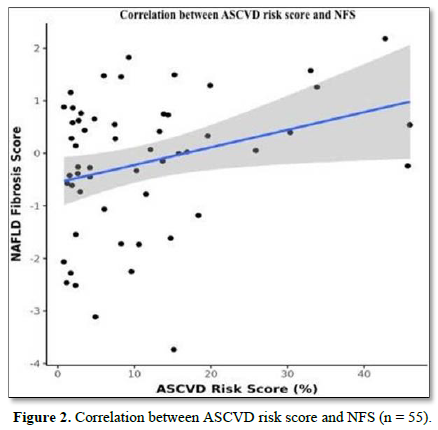
The mean N.F.S. was -0.18 ± 1.30. There was a weak positive correlation between A.S.C.V.D. Risk Score and N.F.S. (ρ = 0.27, p = 0.047), as shown in Figure 2.
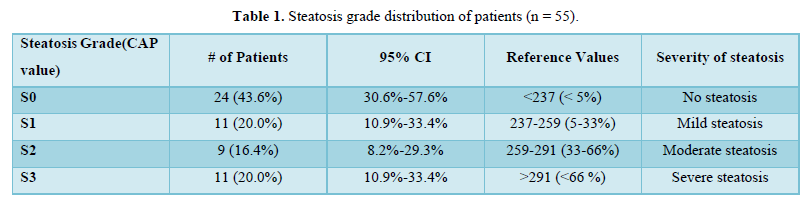
The mean of FibroScan (C.A.P.) was 245.82±50.89. Twenty-four (43.6%) of the patients had steatosis grade S0, 11 (20.0%) of the patients had steatosis grade S1, 9 (16.4%) of the patients had steatosis grade S2 and 11 (20.0%) of the patients had steatosis grade S3 as shown in Table 1.
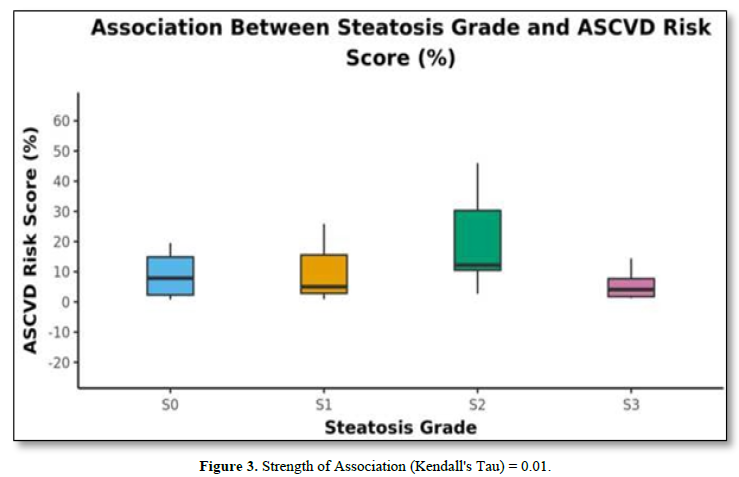
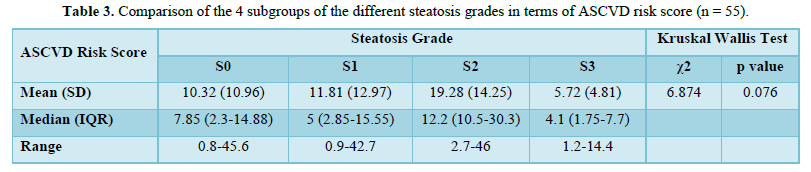
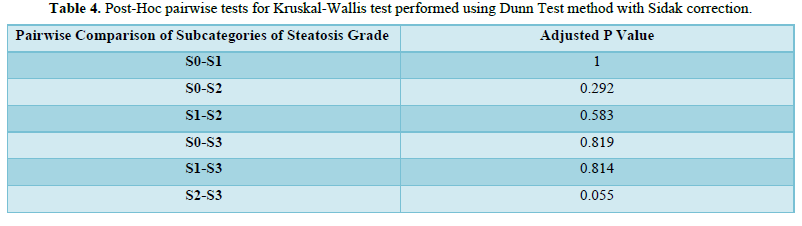
The mean A.S.C.V.D. risk scores in S0, S1, S2, and S3 were 10.32±10.96, 11.81±12.97, 19.28±14.25, and 5.72 ±4.8, respectively, as shown in Table 4. There was no statistically significant difference between the groups regarding the A.S.C.V.D. risk score for the steatosis grade (χ2 = 6.874, p = 0.076). Figure 3 shows the association between steatosis grade and A.S.C.V.D. Risk score.
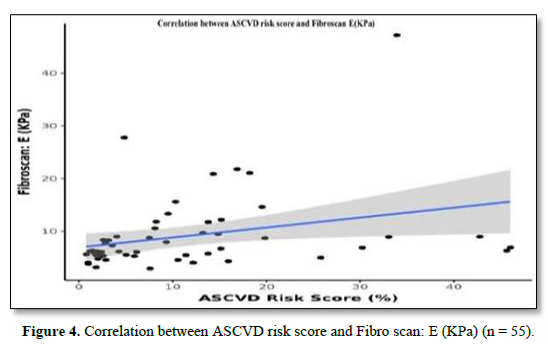
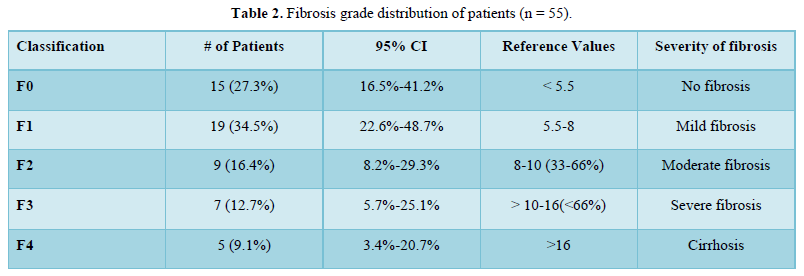
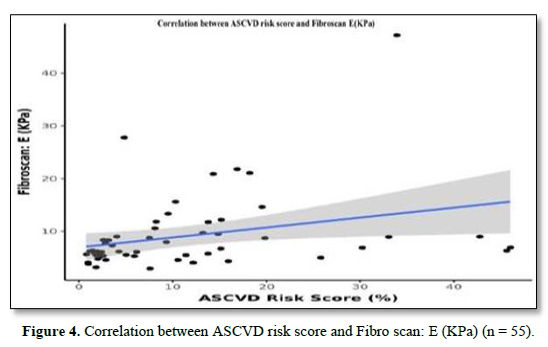
The mean Fibro Scan (kPa) was 9.05±7.24. Fifteen (27.3%) of the patients had a Fibrosis Grade of F0, 19 (34.5%) of the patients had a Fibrosis Grade of F1, 9 (16.4%) of the patients had a Fibrosis Grade of F2, 7 (12.7%) of the patients had Fibrosis Grade of F3 and 5 (9.1%) of the patients had Fibrosis Grade of F4 as shown in Table 2. There was a moderate positive correlation between A.S.C.V.D. risk score and Fibro Scan (kPa), and this correlation was statistically significant (ρ = 0.47, p = <0.001), as shown in Figure 4.
DISCUSSION
Given the strong association between NAFLD and T2DM, assessing the two conditions' independent cardiovascular effects remains challenging. To summarize, our study implemented the use of fifty-five patients, all with known cases of T2DM for at least five years. These patients were evaluated and investigated for NAFLD using a USG of the abdomen, NFS, and Fibro Scan results. ASCVD was calculated and analyzed for association with NAFLD.
Previous studies have shown that NAFLD could be an independent cardiovascular disease risk factor. Examples are as follows:
Targher [2] screened 2839 diabetic patients and found NAFLD patients had remarkably (p<.001) higher rates for the gender-adjusted prevalence of coronary (26.6 vs. 18.31%), cerebrovascular (20% vs. 13.3%), and peripheral vascular disease than their counterparts without NAFLD (15.4 vs. 10%) [2].
Kalra [17] conducted a study to determine frequency and risk factors in Type II DM patients. Out of 924 patients (335 females/569 males) in the age group of 15-85 years were identified as having NAFLD. There was no significant difference in ASCVD scores in fatty and non-fatty liver groups [17].
Tuong [18] conducted a cross-sectional design in T2DM adults. In this study, liver steatosis and fibrosis were assessed by Fibro Scan. NAFLD was diagnosed if CAP > 233 dB/m (steatosis > 5%). They found that 307 T2DM patients qualified for the study's criteria. The prevalence of NAFLD in T2DM patients based on Fibro Scan was 73.3%. Both mild, moderate, and severe steatosis rates were 20.5%, 21.8%, and 30.9%, respectively. The prevalence of significant fibrosis (≥ F2), advanced fibrosis (≥ F3), and cirrhosis (F4) was 13.0%, 5.9%, and 3.6%, respectively. They concluded that their study supports screening for NAFLD and evaluating the severity of liver fibrosis in T2DM patients [18]. The mean Fibro Scan CAP of our study population was 245.82 ± 50.89. 24 (43.6%) of the participants had Steatosis Grade: S0. 11 (20.0%) of the participants had Steatosis Grade: S1. 9 (16.4%) of the participants had Steatosis Grade: S2. 11 (20.0%) of the participants had Steatosis Grade: S3.
There was a significant difference between the two groups in Fibro scan CAP (W = 383.500, p = 0.029), with the median Fibro scan CAP being highest in the fatty liver group. The strength of association was correlated using a Point-Biserial Correlation of 0.3, showing a medium effect size. However, there was no significant difference between the groups regarding ASCVD Risk Score (%) (W = 374.500, p = 1.000).
There was a moderate positive correlation between ASCVD Risk Score (%) and Fibro scan: E (KPa), and this correlation was statistically significant (rho = 0.47, p = <0.001). For every 1 unit increase in ASCVD Risk Score (%), the Fibro scan: E (KPa) increases by 0.19 units. Conversely, for every 1 unit increase in Fibro scan: E (KPa), the ASCVD Risk Score (%) increases by 0.48 units.
Golabi [19] studied the correlation between cardiovascular risk and NAFLD in patients with T2DM. Among 1,262 subjects with NAFLD (47.9% men; 41.2% white; mean age of 56.3 years), the high risk for cardiovascular disease was 55.9%, and 4.8% had advanced fibrosis. After a median follow-up of 17.7 years, 482 subjects (38.2%) died of prevalent causes, of whom 382 (79.3%) had a high risk for cardiovascular disease. The unadjusted overall and cardiac-specific mortality were higher for patients with NAFLD who had an increased risk for cardiovascular disease than subjects with NAFLD with a low risk for cardiovascular disease (57.3% vs. 16.8% for overall mortality; 16.4% vs. 3.5% for cardiovascular mortality). After controlling for risk factors associated with mortality, a higher risk for cardiovascular disease was associated with a 42% higher overall mortality rate (adjusted HR [aHR] of 1.42; 95% confidence interval [CI], 1.05-1.91) and twice the risk of cardiovascular mortality (aHR, 2.02; 95% CI, 1.12-3.65). Adjusted population attributable fractions (PAFs) were 11.4% for overall mortality and 44.9% for cardiovascular mortality [19]. The mean ASCVD Risk Score (%) was 11.16 ± 11.58. The mean (SD) of ASCVD Risk Score (%) in the male subject group was 14.11 (13.54). The mean (SD) of ASCVD Risk Score (%) in the female group was 8.52 (8.93). There was a moderately positive correlation between Fibro Scan E (KPa) and ASCVD Risk Score (%), and this correlation was statistically significant (rho = 0.47, p = <0.001).
In summary, NAFLD is highly prevalent, with a proportion of these patients developing liver disease so timely screening with Transient elastography using Fibro Scan with C.A.P. can assess both liver steatosis and fibrosis simultaneously, which can halt further complications from developing. On the other hand, NAFLD patients succumb to death most commonly due to cardiovascular complications and predictive models, such as A.S.C.V.D. risk score can provide an easy tool to identify patients with NAFLD who are at the greatest risk for CVD NAFLD act as a marker for CVD, and early interventions for it can improve long-term cardiovascular outcomes. Overall, this work can have a significant impact on clinical and public health. However, further research is needed to validate these findings. Although meaningful conclusions may be drawn from this study, a significant limitation of our study is the small sample size, and future studies should be carried out using larger patient populations. In addition, in our study, we did not perform a histopathological examination for confirmation of non-alcoholic steatohepatitis and A.S.C.V.D. risk score is a reliable assessment tool, but its generalizability and applicability in our study of patients are debatable.
CONCLUSION
Our study revealed the Prevalence of fatty liver on abdominal U.S.G. in 72% of the patients (42/55). However, there was no significant difference in A.S.C.V.D. Risk score in both fatty and non-fatty liver groups. Additionally, N.F.S. correlated significantly with the A.S.C.V.D. risk score. There was no significant difference between A.S.C.V.D. Risk score in the steatosis and non-steatosis groups. There was a moderate positive correlation between A.S.C.V.D. the risk score and Fibro Scan E, and this correlation was statistically significant. However, these results suggest that identifying NAFLD in T2DM may help CVD risk prognosis and thus, have substantial management implications. Identifying people with NAFLD would highlight a subgroup of diabetic patients who may benefit from more intensive preventative treatment and cardio-protective measures to decrease their risk of future CVD.
- Kudaravalli P, John S (2022) Nonalcoholic Fatty Liver. [Updated 2022 May 8]. In: StatPearls. Treasure Island (FL):
- Targher G, Bertolini L, Padovani R, Rodella S, Tessari R, (2007) Prevalence of nonalcoholic fatty liver disease and its association with cardiovascular disease among type 2 diabetic patients. Diabetes Care 30(5): 1212-1218.
- Angulo P (2002) Nonalcoholic fatty liver disease. N Engl J Med 346(16): 1221-1231.
- McCullough AJ (2006) Pathophysiology of Nonalcoholic Steatohepatitis. J Clin Gastroenterol 1: S17-S29.
- Marchesini G, Marzocchi R, Agostini F, Bugianesi E (2005) Nonalcoholic fatty liver disease and the metabolic syndrome. Curr Opin Lipidol 16(4): 421-427.
- Day CP (2006) Non-alcoholic fatty liver disease: current concepts and management strategies. Clin Med (Lond) 6(1): 19-25.
- Clark JM, Brancati FL, Diehl AM (2003) The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol 98(5): 960-967.
- Bedogni G, Miglioli L, Masutti F, Tiribelli C, Marchesini G, Bellentani, S (2005) Prevalence of and risk factors for nonalcoholic fatty liver disease: The Dionysos nutrition and liver study. Hepatology 42: 44-52.
- Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, et al. (2004) Prevalence of hepatic steatosis in an urban population in the United States: Impact of ethnicity. Hepatology 40: 1387-1395.
- Caussy C, Aubin A, Loomba R (2021) The Relationship Between Type 2 Diabetes, NAFLD, and Cardiovascular Risk. Curr Diab Rep 21: 15.
- Younossi ZM, Golabi P, de Avila L, Paik JM, Srishord M, et al. (2019) The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. J Hepatol 1(4): 793-801.
- Doycheva I, Cui J, Nguyen P, Costa EA, Hooker J, et al. (2016) Non-invasive screening of diabetics in primary care for NAFLD and advanced fibrosis by MRI and MRE. Aliment Pharmacol Ther 43(1): 83-95.
- Zhou YY, Zhou XD, Wu SJ, Hu XQ, Tang B, et al. (2018) Synergistic increase in cardiovascular risk in diabetes mellitus with nonalcoholic fatty liver disease: A meta-analysis. Eur J Gastroenterol Hepatol 30(6): 631-636.
- Targher G, Lonardo A, Byrne CD (2018) Nonalcoholic fatty liver disease and chronic vascular complications of diabetes mellitus. Nat Rev Endocrinol 14(2): 99-114.
- Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, et al. (2005) Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 41(6): 1313-21.
- Lucey MR, Im GY, Mellinger JL, Szabo G, Crabb DW (2020) Introducing the 2019 American Association for the Study of Liver Diseases Guidance on Alcohol-Associated Liver Disease. Liver Transpl 26(1): 14-16.
- Kalra S, Vithalani M, Gulati G, Kulkarni CM, Kadam Y, Pallivathukkal J, et al. (2013) Study of prevalence of nonalcoholic fatty liver disease (NAFLD) in type 2 diabetes patients in India (SPRINT). J Assoc Physicians India 61(7): 448-453.
- Tuong TTK, Tran DK, Phu PQT, Hong TND, Dinh TC, Chu DT (2020) Non-Alcoholic Fatty Liver Disease in Patients with Type 2 Diabetes: Evaluation of Hepatic Fibrosis and Steatosis Using Fibro scan. Diagnostics (Basel) 10(3): 159.
- Golabi P, Fukui N, Paik J, Sayiner M, Mishra A, Younossi, ZM. (2019) Mortality Risk Detected by Atherosclerotic Cardiovascular Disease Score in Patients with Nonalcoholic Fatty Liver Disease. Hepatol Commun 3(8): 1050-1060.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Stem Cell Research and Therapeutics (ISSN:2474-4646)
- International Journal of Anaesthesia and Research (ISSN:2641-399X)
- Journal of Alcoholism Clinical Research
- Journal of Renal Transplantation Science (ISSN:2640-0847)
- Dermatology Clinics and Research (ISSN:2380-5609)
- Journal of Spine Diseases
- Ophthalmology Clinics and Research (ISSN:2638-115X)